High-Temperature Fuel Cells for Mobile and Stationary Applications
Intermediate-Temperature Water Splitting Using Protonic Ceramic Electrolysis Cells
US Department of Energy’s Fuel Cell Technologies Office (FCTO) has funded this 3-year $1.5M project to advance the development of a hydrogen-focused integrated renewable energy production. This project features R&D of first of kind intermediate temperature mixed defect conducting protonic ceramic electrolysis cell (PCEC) technology that has the ability to integrate renewable electricity and excess process heat for cost effective hydrogen production. In collaboration with the CSM Profs. Ryan O’Hayre, Neal Sullivan and Fuel Cell Energy, our team is developing a mixed defect conducting electrolyte that operates at an intermediate temperature range (500°C – 700°C) with high faradaic efficiency generating high-quality hydrogen utilizing low-cost materials[1,2]. In particular, our research group focuses on electrolysis cell modeling, utility-scale system designing and optimizing, evaluating the economic performance of the system, and analyzing system life cycle emissions.
PCEC is composed of dominantly proton-conducting barium zirconate-cerate oxide co-doped with ytterbium and yttrium (commonly referred to as BCZYYb) electrolyte layer that is sandwiched between triple conducting steam electrode (BCFZY) and thin Ni-BZY20 hydrogen electrode. Modeling PCEC performance is challenging because of the factors like multiple charge carrier movements, open circuit voltage (OCV) reduction, and faradaic efficiency variation. We have developed a framework[3] for a computationally efficient, high fidelity, steady-state, heterogeneous, cell-level, interface charge transfer model for PCEC that couples the conservation equations with the Nernst-Planck[4] formulation.
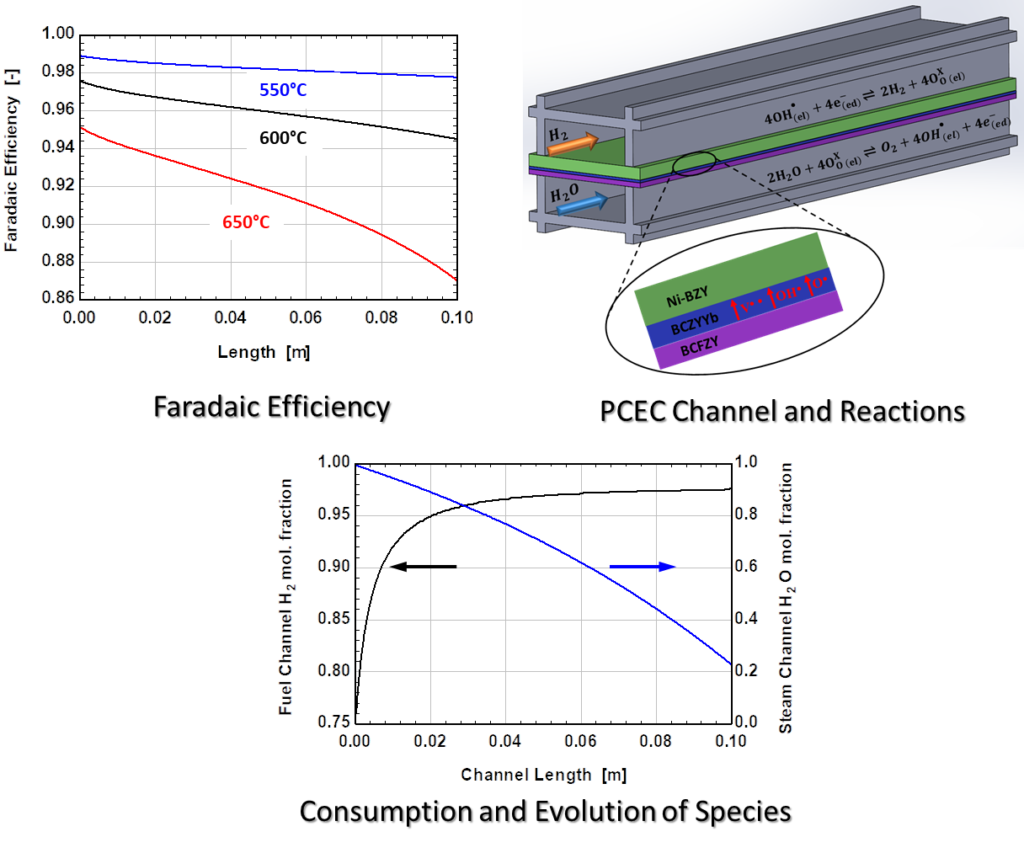
We formulated the model in a way that reduces the empiricism, allows for easy integration of modeling parameters extracted from button cell experiments, and enables rapid performance scale-up to cell-level predictions. We simulated the performance of electrolysis cells based on model under various thermal gradients, inlet compositions, and cell voltages boundary conditions to predict the distribution of properties like species concentration, pressure, temperature, and faradaic efficiency along the length of cell channel. We are designing PCEC-based utility-scale hydrogen production plant targeting 50 ton per day supply of pressurized hydrogen (20 bar) that operates at an overall efficiency exceeding 65%. We also estimate using H2A tool developed by EERE that the cost of hydrogen production using PCEC system is on par with ‘Current and Future’ solid oxide electrolysis system and is better than ‘Current and Future’ proton exchange membrane system.
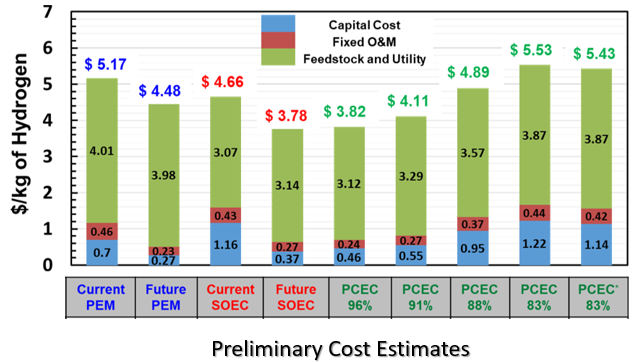
Our efforts are one step towards the economic conversion of renewable electricity to hydrogen which has the potential to limit the renewable curtailment, provide positive economic impact, enhance grid flexibility, increase energy security and reduce emissions.
FUNDING: DOE Fuel Cell Technologies Office
References
[1] C. Duan, R. Kee, H. Zhu, N. Sullivan, L. Zhu, L. Bian, D. Jennings, R. O’Hyare, Highly efficient protonic ceramic electrochemical cells for power generation and fuel production, Nature Energy 4 (3) (2019) 230-240.
[2] A. Dubois, S. Ricote, and R. Braun, Benchmarking the expected stack manufacturing cost of next generation, intermediate-temperature protonic ceramic fuel cells with solid oxide fuel cell technology, J. Power Sources 369 (2017) 65-77.
[3] A. Thatte, R. Braun, and R. Kee, Steady-state modeling of multiple defect conducting protonic ceramic electrolysis cells using Nernst-Planck formulation, J. Electrochem. Soc., In publication
[4]H. Zhu, S. Ricote, C. Duan, R. O’Hyare, R. Kee, Defect incorporation and transport within dense BCZYYb proton-conducting membranes, J. Electrochem. Soc. 165 (10) (2018) F845-F853
Current Projects
Past Projects
Selected Publications in This Research Area
No Results Found
The page you requested could not be found. Try refining your search, or use the navigation above to locate the post.
View Other Research Areas:
MODELING AND SYSTEMS ANALYSIS OF ALTERNATIVE FUEL PRODUCTION AND UTILIZATION SYSTEMS
RENEWABLES AND GRID-ENERGY STORAGE SYSTEMS
